If you are currently following a ketogenic diet, also called keto, then you have probably…

A Practical Water Guide for Coffee Professionals (Part I)
With the emergence of science-based resources like the SCA’s Water Quality Handbook and the Maxwell Colonna-Dashwood and Christopher Hendon collaboration Water for Coffee, baristas and roasters are more aware than ever of the critical role of water in coffee quality.
Rather than merely prescribing a formula, we’ve always found it more enlightening to experiment with possibilities, and therefore some colleagues and I have been tinkering with water, and investigating what kinds of flavors might spring up.
Before heading downstream in Part II of this two-part series, which will dive into sensory data, Part I will solidify some H2O basics, and get to the source of our brewing possibilities. We’ll also look at some practical methods for testing and preparing your water.
Water is so much more than just two parts of hydrogen to one part oxygen. This seemingly simple compound provides a playground for all kinds of complex chemical interactions.
Acidification & the Bicarbonate Cycle
An important example of water’s complexity can be seen in its molecular structure. The bond between the elements in H2O is somewhat weak, and when water comes in contact with atmospheric carbon dioxide, a little of the CO2 wedges its way into the equation, creating carbonic acid (H2CO3). This is in part why some dentists caution against drinking too much carbonated water; it’s acidic — though it’s probably not that big of a deal, especially if unflavored.
This is just the first step in a process called the bicarbonate cycle, where a positively charged hydrogen ion (H+) in the carbonic acid “dissociates” and leaves us with the negatively charged base bicarbonate (HCO3–). This happens in your bloodstream all the time; the weak acid and base balance is great at buffering water against changes in pH. Because your blood has a very narrow window (7.35 – 7.45 pH) to ensure your survival, it uses this bicarbonate cycle to manage the introduction of acids and bases into your system and keep you alive.
In water, bicarbonate concentration can be referred to as alkalinity, and it’s important to have at least a little around to prevent violent swings in pH, a capacity referred to as buffering. (Side note: Don’t confuse the term “alkalinity” with “alkaline,” which is used to describe substances that have a high [base] pH.)
Why might the pH of water change? H2O in its pure state will bond readily with all kinds of materials, like CO2, which can dramatically impact pH. Just look at the acidification of global water supply as an example.
Mineral Content & Total Dissolved Solids
Limestone presents us with an alternative example of water’s absorbing capacity. As rainwater flows across the ground or through underground passages, it picks up calcium, in the form of calcium carbonate (CaCO3). In and of itself, calcium carbonate isn’t all that soluble, but when it meets up with carbon dioxide in water, it creates calcium bicarbonate, which is very soluble. When CO2 levels drop, calcium bicarbonate will leave the water and cling to whatever it happens to be in contact with: hot water boilers, pipes, faucets, you name it. We call this limescale.
Calcium from limestone is one of the most common hard minerals present in water. Magnesium ions also occur naturally. Additionally, you can often find trace amounts of iron, lead, copper, silica, and aluminum, sometimes leached from pipes and storage tanks. Other mineral salts might include sodium and potassium bicarbonates, which frequently act as buffers, sometimes present as a result of softening treatments. Each of these will contribute to the Total Dissolved Solids count (TDS), a number that is also sometimes expressed as grains of hardness. One grain is about 17.1 parts per million in TDS.
But that’s hardly the end of it. H2O is frequently awash in trace contaminants like nitrites and nitrates from fertilizers or fish poop, chloramines and fluoride from treatment, sulfates, chlorates, and a host of other undesirables. If you’re in most cities in the US, you ought to be able to access this information; water utility companies are required to provide quarterly summaries and annual reports. Royal’s office and the Crown are fed by sources managed by EBMUD, and their data on the water from the reservoirs and treatment plants they operate is available online here, by way of example.
Some of this can be measured by titration, and you can get a broad glimpse of a water’s constitution by using an electronic conductivity meter (sometimes erroneously referred to as TDS meters). However, for the majority, the most accurate way to know what’s in your water is to send it off to a lab. If you’re looking to purchase new equipment, like an espresso machine, oftentimes manufacturers will offer to test your water for you, since it’s in their interest to prevent long-term damage to the equipment.
Water’s complicated constitution requires some management options. There are essentially three things to keep in mind when choosing a water formulation: longevity of brewing equipment, sensory quality, and drinking safety.
Keeping the water at a relatively neutral pH — some people tend to prefer slightly alkaline — and a balanced hardness will help to preserve boilers, espresso machines and other equipment. A little bit of bicarbonate alkalinity can act as a buffer against swings in pH, which is good since water can be corrosive without bicarbonate.
Water for Coffee has made a pretty clear case for the benefits of calcium and magnesium ions in extracting coffee efficiently. Hendon and Colonna-Dashwood assert that magnesium is a little more efficient, they seem to prefer its flavor, and it has the added benefit of not resulting in limescale, as calcium is prone to do. However, calcium is present naturally in greater quantities than magnesium. The SCA’s water specification uses calcium as their benchmark for hard mineral content.
What remains — typically chloramines and nitrates and some other trace contaminants — can generally be filtered using an active carbon block. This is a great first step in filtration – it keeps your water safe to drink and doesn’t remove the minerals we’re interested in retaining. It virtually eliminates anything that might affect the color (“turbidity”) and odor of the water. In my opinion, everyone should have one for drinking water.
What’s next will depend quite a bit on your ideal for sensory and your local source composition.
Hard Water Solutions
Places with hard water, above 150-200 ppm general hardness, will likely benefit from some form of mineral reduction. A few accessible options exist here; the two most common are ion exchange and reverse osmosis.
Cartridge systems can provide varying degrees of ion exchange, a system that passively ‘trades’ hard mineral ions for charged sodium or potassium salts. This typically increases the buffering alkalinity of a water, as well, and may lead to increased saltiness. However, many modern systems are highly programmable and precise. They have the added benefit of taking up relatively little space and producing little to no waste aside from regularly scheduled cartridge replacements.
Reverse osmosis uses a membrane to reduce the total dissolved content of water to very nearly zero. The resulting “empty” water is fed into a holding tank, while the minerals and other compounds blocked by the membrane must be flushed away. Most RO units will then reintegrate a portion of carbon-filtered water back into the demineralized water to achieve a target TDS. An independent pump and custom water lines are usually required to provide adequate line pressure and appropriate pathways to deliver the water to its intended destination.
If your water is especially hard, RO can provide an effective way to reduce its mineral content. However, there are some flaws. The amount of water required to flush the membrane, which goes down the drain (or, at best, can be reused as gray water), is significant. Factory settings for most RO systems can be in the neighborhood of 4 flushed gallons for every 1 gallon that’s kept. This is unfortunate, given that many places with especially hard water are also places prone to drought or otherwise restricted access to fresh, clean water. Draining away significant amounts of clean, treated (albeit hard) water may be a problem of its own.
The other potential complication with RO is that by blending a percentage of the source water back into the solution, you’re not really solving mineral imbalance problems. The water you’ve created is just a diluted version of what already exists. So, if your water is proportionally high in alkalinity, for example, RO water will not change that.
Soft Water Solutions
In soft water scenarios, at or below 50 ppm general hardness, some form of remineralization may be in order, both to help coffee extract in ways that are delicious, but also to prevent the possibility of equipment corrosion due to low alkalinity.
This is a little more challenging than reducing minerals in hard water. There are cartridge options that exist; these are frequently some type of calcium or magnesium (crushed coral is a common option) that water flows over to add healthy hard minerals. In most cases, these cartridges depend on contact time. Thus if the water is streaming at a constant rate through the system, relatively little of the mineral will dissolve. Conversely, if the water sits for a long time in the cartridge, it can result in a sudden spike in mineral content.
Systems exist that mineralize water; notably Global Customized Water offers options to inject their proprietary liquid formula in precise increments to optimize water for coffee (typically in the form of RO followed by liquid mineral addition). Third Wave Water has a similar, manual option that can mineralize distilled or deionized water in small batches by using pre-formulated dry mineral packets.
Ad hoc mineral addition is also possible. I’ve been experimenting with this a bit, and it requires a lot of attention to detail, an extremely precise scale, some moderate safety precautions, and some pretty good math. It does allow for highly customized water formulas, however. I keep sodium bicarbonate (baking soda) and potassium bicarbonate on hand for my alkalinity buffers. For minerals, the options include calcium and magnesium chlorides and sulfates. The sulfate forms I’ve found to be less readily soluble in water, often leaving a chalky, undissolved residue, so I tend to use the chlorides. Each of these minerals are easily available and inexpensive.
If you plan on mineralizing water manually, or you wish to better understand the charts and language used by the industry, there’s a highly technical but critical note to make sense of the work.
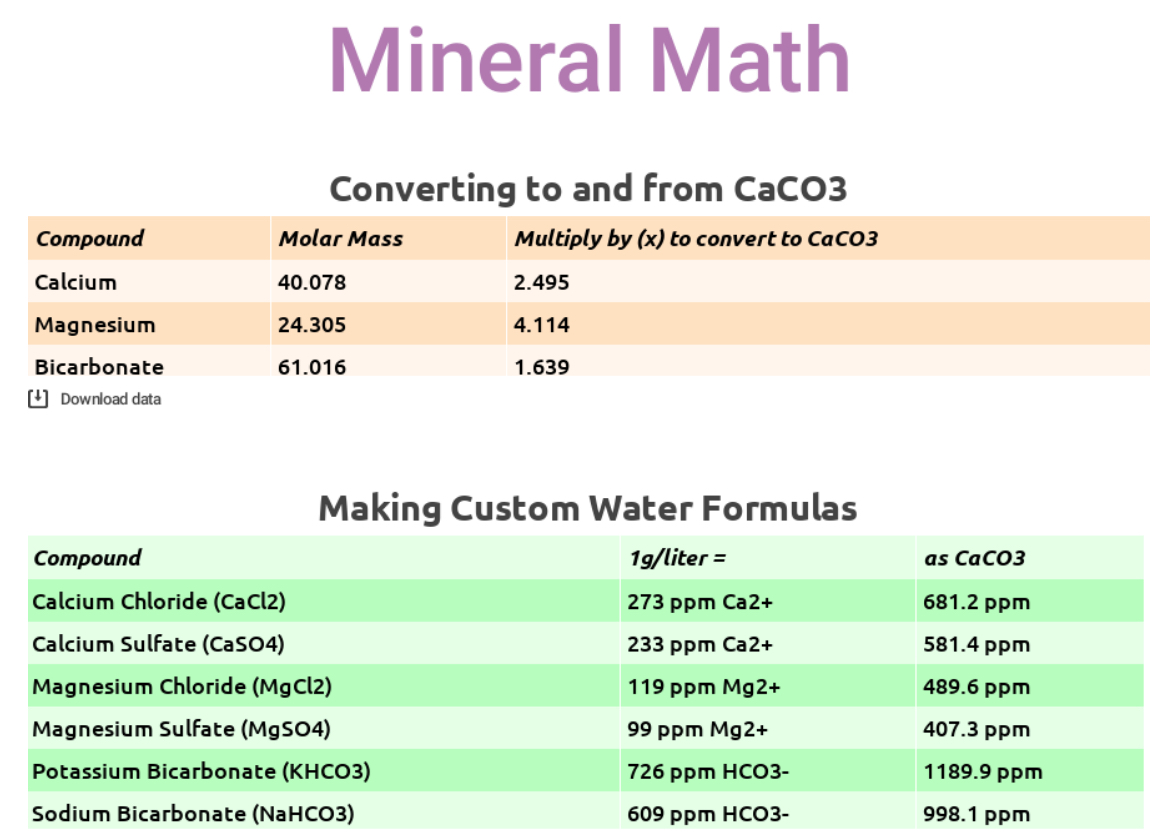
Most people record the quantity of minerals in water using measurements in parts per million (ppm), and most scientists still use calcium carbonate (CaCO3) as the standard for measuring the concentration of any compound in water. This means that the minerals we’re concerned with are usually reported in ppm as something other than what they actually are.
We can decode this confusing language with a little math. Stick with me.
The reason CaCO3 is the reporting unit has to do with its convenient molar mass: 100 grams. You might recall from high school chemistry class that a mole is just a number: 6.022 x 1023 to be precise. It is the common denomination that scientists use to count molecules. In one mole of CaCO3 there is almost exactly 100g of mass. Which is convenient but not intuitive when we’re talking about literally anything other than calcium carbonate. You might’ve noticed this in the SCA Water Quality Handbook or in the Corrigendum to Water for Coffee, where confusion has arisen as a result of inconsistent reporting conventions.
A Practical Example
Let’s say I’ve added 1 g of magnesium chloride (MgCl2) to 1 liter of water. Available as a hexahydrate (MgCl2 * 6H2O), by mass magnesium chloride is roughly 11.9% magnesium ion, which should result in the addition of around 119 ppm magnesium ion (Mg2+) to the water. The math for this is simple: 1 g/L x 11.9% = 0.119 g/L = 119 ppm Mg2+. (We’ve also added about 348 ppm chloride, which is problematic for other reasons; I’ll address this a little later… just remember 119 ppm magnesium for now.)
Mg2+ has a molar mass of 24.305 g/mol, meaning it is significantly less dense than CaCO3’s 100g/mol. But to report Mg2+ as CaCO3, we must pretend that the magnesium ions are the same mass as calcium carbonate. How? Well, 100g/mol ÷ 24.305g/mol = 4.11. This means that magnesium is 4.11 times less dense than calcium carbonate.
So, just like a good scientist, to pretend that Mg2+ is CaCO3, multiply our 119 ppm of magnesium by 4.11. Thus 119 ppm Mg2+, when reported as CaCO3 is roughly 489 ppm.
To reiterate and clarify: the ratio of magnesium ions to water in this example is 119 Mg2+ per million H2O. But scientists convert, pretending that magnesium is calcium carbonate. If magnesium were calcium carbonate, then there would be 489 ppm Mg2+ in the solution.
Confusing, yes, but important as we start to talk about mineral concentrations below, because clearly 119 is not the same number as 489. How we report concentration makes a difference.
Here are some quick and dirty conversions for you, when using magnesium, calcium, and bicarbonates to create custom water:
Using the math in these tables, you can fairly quickly decipher some formulas for creating custom water. Let’s say you want to make 4 liters of water with 80 ppm hard minerals (as CaCO3), equal parts calcium and magnesium chlorides, and 40 ppm alkalinity (as CaCO3) using potassium bicarbonate, a common and easy to work with spec that falls inside the current SCA & Water for Coffee “optimal” zones. Here’s the math:
- 40ppm Ca2+ (as CaCO3) ÷ 681.2 = 0.0587 g x 4L = 0.235 g of CaCl2 to add to 4L water
- 40ppm Mg2+ (as CaCO3) ÷ 489.6 = 0.0817 g x 4L = 0.327 g of MgCl2 to add to 4L water
- 40ppm HCO3– (as CaCO3) ÷ 998.1 = 0.040 g x 4L = 0.160 g of KHCO3 to add to 4L water
Note how little of these chemicals you need to create mineralized water.
Ok, now let’s address the chloride problem. Chlorides — not to be confused with chlorine, the chloride ion is recommended in small amounts for consumption — and, to a lesser degree, sulfates, raise the conductivity of water and are corrosive to metals under certain conditions such as low pH, high pressure, and/or high temperature. Espresso machine manufacturers, for example, set stringent limits for chloride content and conductivity in the water. So take care when using these minerals in high concentrations.
In our example from earlier, the addition of 1g of MgCl2 resulted in very high concentrations of both magnesium and chloride. But reduce that 0.1g of MgCl2 and we’ve added a reasonable amount of magnesium (about 49ppm as CaCO3) and a safe level of chlorides, well under 200 ppm, for low grade stainless steel. Under heat and pressure, however, chloride’s corrosive properties are enhanced, so even the 35 ppm exceeds La Marzocco’s espresso machine specifications, for example.
This seems like as good a time as any to take a deep breath and regroup.
We’ve looked at the basics of how water behaves, and at all the kinds of minerals, salts, and other compounds that might be swimming around in your H2O. We’ve also looked at some practical solutions for managing the water coming out of your taps — basics like filtration and advanced customization. And we took an extended detour into the seedy underbelly of molar mass conversions.
In the next installment, we’ll look at some specific sensory data and information related to alkalinity and mineralization of water.
Chris Kornman
Chris Kornman is a seasoned coffee quality specialist, avid writer and researcher, and the Lab & Education Manager at The Crown: Royal Coffee Lab & Tasting Room.